 These are Online Notes on "Haloalkanes and Haloarenes" (Chapter=>10 ) Part 1 for practice of CBSE BOARD, CBSE NEET, CSIR NET Chemical Sciences etc.
These are Online Notes on "Haloalkanes and Haloarenes" (Chapter=>10 ) Part 1 for practice of CBSE BOARD, CBSE NEET, CSIR NET Chemical Sciences etc.
1. Nature
of C-X bond in alkyl halides: X is more electronegative than carbon. So, the
C-X bond is polarized with C having a partial
positive charge and X having a partial negative charge.
2. Preparation
of haloalkanes:
3.
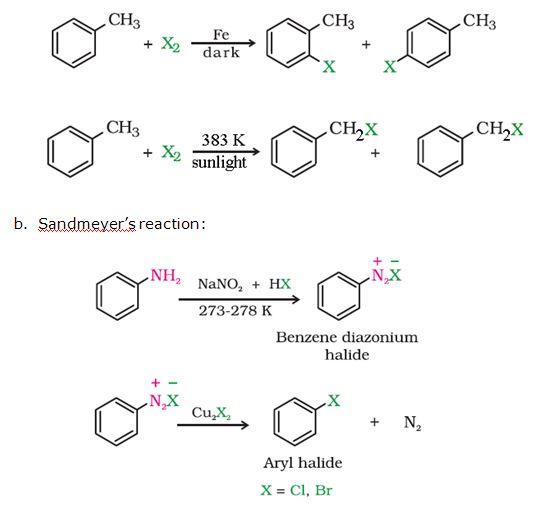
Preparation of
haloarenes:
a. By
elecrophilic substitution reaction:
4.
Physical properties of haloalkanes:
a.
Solubility. Although haloalkanes are polar in nature,
yet they are practically very slightly soluble in water. In order for a haloalkane to dissolve in
water, energy is required to overcome the attractions between the haloalkane
molecules and break the hydrogen bonds between water molecules. However
Haloalkanes are not able to form hydrogen bonds with water and therefore, less
energy is released when new attractions are set up between the haloalkane and
the water molecules because these are not as strong as the original hydrogen
bonds in water molecules. As a result, solubility of haloalkanes in water is low.
b. Density:
·
Simple fluoro and chloroalkanes are lighter than
water while bromides and polychloro devrivatives are heavier than water.
·
With the increase in number of carbon atoms, the
densities go on increasing.
·
With the increase in number of halogen atoms,
the densities go on increasing.
·
The densities increase in the order:
·
Fluoride < chloride < bromide < iodide
·
The density also increases with increasing
number and atomic mass of the halogen.
b. Method
and Boiling points:
·
Molecules of organic halogen compounds are generally polar.
Due to the
polarity as well as higher molecular mass as compared to the parent
hydrocarbon, the intermolecular forces of attraction (dipole – dipole and van
der Waals) between the molecules are stronger in halogen derivatives of
alkanes. As a result melting and boiling points of chlorides, bromides and
iodides are considerably higher than those of the parent hydrocarbon of
comparable molecular mass.
·
Amongst themselves, the following trends are observed:
o
For the same alkyl group the boiling points of
alkyl chlorides, bromides and iodides follow the order RI > RBr
> RCl > RF where R is an alkyl group. This is because with the
increase in the size of the halogen, the
magnitude of van der Waals force increase.
o
In general,
the boiling points of chloro, bromo and iodo compounds increase with increase
in the number of halogen atoms.
o
For the same halogen atom, the boiling points of
haloalkanes increase with increase in the size of alkyl groups.
o
For isomeric alkyl halides, the boiling points
decrease with branching. This is because branching of the chain makes the
molecule more compact and, therefore, decrease the surface area. Due to
decrease in surface area, the magnitude of van der Waals forces of attraction
decreases and consequently, the boiling points of the branched chain compound is less than those of the
straight chain compounds.
5. Physical
Properties of Haloarenes:
a. These
are generally colourless liquids or crystalline solids.
b. These
are heavier than water.
c. Melting
and boiling points of haloarenes:
·
Melting and boiling points of haloarenes are
nearly the same as those of alkyl halides containing the same number of carbon atoms.
·
The boiling points of monohalogen derivatives of
benzene are in the order: iodo > bromo > chloro > fluoro
·
For the same halogen atom, the melting and
boiling points increase as the size of the aryl group increases.
·
The melting point of para isomer is quite higher
than that of ortho or meta isomers. This is due to the fast that is has
symmetrical structure and there fore, its molecules can easily pack loosely in the crystal lattice.
As a result intermolecular forces of attraction are stronger and therefore, greater energy is required to
break its lattice and it melts at higher temperature.
5.
Chemical properties of haloalkanes:
a. Nucleophilic
substitution reaction:
When a
haloalkane with β-hydrogen atom is heated with alcoholic solution of potassium
hydroxide, there is elimination of hydrogen atom from β-carbon and a halogen
atom from the α-carbon atom. As a result, an alkene is formed as a product.
Zaitsev
rule (also pronounced as Saytzeff) is followed.It states that “In
dehydrohalogenation reactions, the preferred product is that alkene which has
the greater number of alkyl groups attached to the doubly bonded carbon atoms.”
a.
Reaction with metals:
·
Reaction with
Magnesium: Grignard’s reagent is formed:
R - X +Mg ¾¾dr¾y et¾he¾r ¾® RMgX
·
Wurtz reaction:
R-X + 2 Na + X-R → R-R + 2 NaX








No comments:
Write comments