These are "H-Bonding, Short Notes and Questions Answers" of chemical bonding chapter of NCERT book in CBSE BOARD.
Points on "Hydrogen Bonding" are as follows:
>Hydrogen bonds are primarily electrostatic and are formed with both strong and weak donors and acceptors.
> A hydrogen bond is an interaction between a proton donor group D-H and a proton acceptor atom A, the D-H…A interaction being called as a hydrogen bond.
> Generally, a hydrogen bond can be characterized as a proton shared by two lone electron pairs.
>Hydrogen bond energies range from about 15-40 kcal/mol for strong bonds, 4-15 kcal/mol for moderate bonds and 1-4 kcal/mol for weak bonds.
>The distance between the H and A in a hydrogen bond is less than the sum of their respective vander Waals radii.
>Hydrogen bonds can be experimentally investigated by a variety of experimental techniques, such as Neutron diffraction, X-ray diffraction, NMR, IR and other spectroscopic techniques.
Animation of H-Bonding in H2O Molecule

REFRESH Page To Play it again:
Questions And Answers on H-Bonding:
Q.1. PH3 has lower boiling point than NH3. Why?
Ans: Unlike NH3, PH3 molecules are not associated through hydrogen bonding in liquid state. That is why the boiling point of PH3 is lower than NH3.
Q.2. Why is H2O a liquid and H2S a gas ?
Ans: Because of small size and high electro negativity of oxygen, molecules of water are highly associated through hydrogen bonding resulting in its liquid state.
Q.3. Explain why in spite of nearly the same electro negativity, oxygen forms Hydrogen bonding while chlorine does not.
Ans: Due to small size of Oxygen as compared to Sulphur.
Q.4. Define hydrogen bond. Is it weaker or stronger than the van der Waals forces?
Ans: A hydrogen bond is defined as an attractive force acting between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule (may be of the same kind).
There are two types of H-bonds:
=> Intermolecular H-bond e.g., HF, H2O etc.
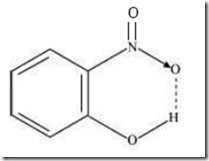
=> Intramolecular H-bond e.g., o-nitrophenol
SHARE this post on FACEBOOK, TWITTER etc.


No comments:
Write comments