ONE MARK QUESTIONS
Q.1. What is the total number of sigma and pi bonds in the following molecules?
(a) C2H2 (b) C2H4
Ans- there are three sigma and two pi-bonds in C2H2. There are five sigma bonds and one pi-bond in C2H2.
Q.2. Write the significance of a plus and a minus sign shown in representing the orbitals.
Ans- Molecular orbitals are represented by wave functions. A plus sign in an orbital indicates a positive wave function while a minus sign in an orbital represents a negative wave function.
Q.3. How do you express the bond strength in terms of bond order?
Ans- Bond strength represents the extent of bonding between two atoms forming a molecule. The larger the bond energy, the stronger is the bond and the greater is the bond order.
Q.4. Define the bond length.
Ans- Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
Q.5. Arrange the bonds in order of increasing ionic character in the molecules: LiF, K2O, N2, SO2 and ClF3.
Ans- N2 < SO2 < ClF3 < K2O < LiF.
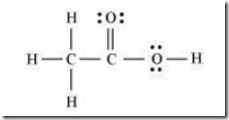
Q.6. The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.

Ans- The correct Lewis structure for acetic acid is as follows:
Q.7. Define octet rule.
Ans- The elements tend to adjust the arrangement of their electrons in such a way that they (except H and He) achieve eight electrons in their outermost shell. This is called octet rule.
Q.8. Define lattice enthalpy.
Ans- The energy required when one mole of an ionic compound in crystalline form is split into the constituent ions is called lattice enthalpy.
Q.9. Which type of bond is formed when the atoms have zero difference in electronegativity?
Ans- Covalent bond.
Q.1. What is the total number of sigma and pi bonds in the following molecules?
(a) C2H2 (b) C2H4
Ans- there are three sigma and two pi-bonds in C2H2. There are five sigma bonds and one pi-bond in C2H2.
Q.2. Write the significance of a plus and a minus sign shown in representing the orbitals.
Ans- Molecular orbitals are represented by wave functions. A plus sign in an orbital indicates a positive wave function while a minus sign in an orbital represents a negative wave function.
Q.3. How do you express the bond strength in terms of bond order?
Ans- Bond strength represents the extent of bonding between two atoms forming a molecule. The larger the bond energy, the stronger is the bond and the greater is the bond order.
Q.4. Define the bond length.
Ans- Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
Q.5. Arrange the bonds in order of increasing ionic character in the molecules: LiF, K2O, N2, SO2 and ClF3.
Ans- N2 < SO2 < ClF3 < K2O < LiF.
Q.6. The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.

Ans- The correct Lewis structure for acetic acid is as follows:

Q.7. Define octet rule.
Ans- The elements tend to adjust the arrangement of their electrons in such a way that they (except H and He) achieve eight electrons in their outermost shell. This is called octet rule.
Q.8. Define lattice enthalpy.
Ans- The energy required when one mole of an ionic compound in crystalline form is split into the constituent ions is called lattice enthalpy.
Q.9. Which type of bond is formed when the atoms have zero difference in electronegativity?
Ans- Covalent bond.


No comments:
Write comments